3. 结构
3.1 二维结构
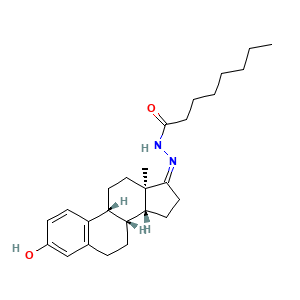
3.2 三维结构
-1
-2
-3
68 71 0 1 0 0 0 0 0999 V2000
3.9392 4.9853 1.4470 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.1414 -3.1858 0.1325 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.4680 -3.1452 0.7620 N 0 0 0 0 0 0 0 0 0 0 0 0
-1.2215 -2.0236 0.7153 N 0 0 0 0 0 0 0 0 0 0 0 0
2.6299 -2.1191 -0.6219 C 0 0 1 0 0 0 0 0 0 0 0 0
1.3837 -1.5972 0.1295 C 0 0 1 0 0 0 0 0 0 0 0 0
3.6305 -1.0231 -1.0226 C 0 0 1 0 0 0 0 0 0 0 0 0
2.8848 0.2547 -1.5571 C 0 0 1 0 0 0 0 0 0 0 0 0
0.5731 -0.6861 -0.8030 C 0 0 0 0 0 0 0 0 0 0 0 0
3.0964 -3.3359 0.1739 C 0 0 0 0 0 0 0 0 0 0 0 0
1.4189 -0.0792 -1.9390 C 0 0 0 0 0 0 0 0 0 0 0 0
0.7717 -2.9473 0.4636 C 0 0 0 0 0 0 0 0 0 0 0 0
4.7244 -0.7523 0.0218 C 0 0 0 0 0 0 0 0 0 0 0 0
1.7819 -4.0725 0.4567 C 0 0 0 0 0 0 0 0 0 0 0 0
1.6391 -0.9086 1.4801 C 0 0 0 0 0 0 0 0 0 0 0 0
3.1037 1.5036 -0.7109 C 0 0 0 0 0 0 0 0 0 0 0 0
5.3937 0.5979 -0.1939 C 0 0 0 0 0 0 0 0 0 0 0 0
4.3540 1.6795 -0.0804 C 0 0 0 0 0 0 0 0 0 0 0 0
2.1545 2.5303 -0.5983 C 0 0 0 0 0 0 0 0 0 0 0 0
4.6312 2.8512 0.6372 C 0 0 0 0 0 0 0 0 0 0 0 0
2.4320 3.6890 0.1319 C 0 0 0 0 0 0 0 0 0 0 0 0
3.6689 3.8504 0.7472 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.5619 -2.1338 0.3892 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.2551 -0.7820 0.4128 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.7410 -0.8457 0.0593 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.4213 0.5233 0.1650 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.9158 0.4864 -0.1693 C 0 0 0 0 0 0 0 0 0 0 0 0
-7.6034 1.8473 -0.0402 C 0 0 0 0 0 0 0 0 0 0 0 0
-9.0929 1.7482 -0.3727 C 0 0 0 0 0 0 0 0 0 0 0 0
-9.7893 3.0906 -0.2155 C 0 0 0 0 0 0 0 0 0 0 0 0
2.2782 -2.5446 -1.5792 H 0 0 0 0 0 0 0 0 0 0 0 0
4.1671 -1.4304 -1.8944 H 0 0 0 0 0 0 0 0 0 0 0 0
3.3741 0.5117 -2.5103 H 0 0 0 0 0 0 0 0 0 0 0 0
0.1196 0.1464 -0.2510 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.2384 -1.2243 -1.3058 H 0 0 0 0 0 0 0 0 0 0 0 0
3.5854 -3.0574 1.1128 H 0 0 0 0 0 0 0 0 0 0 0 0
3.7918 -3.9621 -0.3935 H 0 0 0 0 0 0 0 0 0 0 0 0
1.4495 -0.8075 -2.7624 H 0 0 0 0 0 0 0 0 0 0 0 0
0.8999 0.7857 -2.3664 H 0 0 0 0 0 0 0 0 0 0 0 0
5.4805 -1.5457 -0.0284 H 0 0 0 0 0 0 0 0 0 0 0 0
4.3156 -0.7852 1.0372 H 0 0 0 0 0 0 0 0 0 0 0 0
1.7992 -4.5977 1.4145 H 0 0 0 0 0 0 0 0 0 0 0 0
1.5303 -4.7704 -0.3478 H 0 0 0 0 0 0 0 0 0 0 0 0
2.1670 0.0387 1.3627 H 0 0 0 0 0 0 0 0 0 0 0 0
2.2390 -1.5339 2.1507 H 0 0 0 0 0 0 0 0 0 0 0 0
0.7242 -0.6793 2.0335 H 0 0 0 0 0 0 0 0 0 0 0 0
5.8707 0.6499 -1.1796 H 0 0 0 0 0 0 0 0 0 0 0 0
6.1773 0.7322 0.5607 H 0 0 0 0 0 0 0 0 0 0 0 0
1.1834 2.4606 -1.0773 H 0 0 0 0 0 0 0 0 0 0 0 0
5.6011 2.9762 1.1129 H 0 0 0 0 0 0 0 0 0 0 0 0
1.6774 4.4676 0.2063 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.8318 -1.1399 1.0053 H 0 0 0 0 0 0 0 0 0 0 0 0
4.8421 4.9284 1.8041 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.1329 -0.3599 1.4179 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.7366 -0.1201 -0.2914 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.8586 -1.2380 -0.9584 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.2462 -1.5539 0.7278 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.9197 1.2286 -0.5091 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.2922 0.9114 1.1829 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.0438 0.1114 -1.1923 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.4114 -0.2324 0.4952 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.1212 2.5674 -0.7121 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.4777 2.2240 0.9821 H 0 0 0 0 0 0 0 0 0 0 0 0
-9.5741 1.0159 0.2859 H 0 0 0 0 0 0 0 0 0 0 0 0
-9.2218 1.3933 -1.4019 H 0 0 0 0 0 0 0 0 0 0 0 0
-9.3521 3.8397 -0.8833 H 0 0 0 0 0 0 0 0 0 0 0 0
-10.8522 2.9971 -0.4592 H 0 0 0 0 0 0 0 0 0 0 0 0
-9.7075 3.4570 0.8128 H 0 0 0 0 0 0 0 0 0 0 0 0
1 22 1 0 0 0 0
1 53 1 0 0 0 0
2 23 2 0 0 0 0
3 4 1 0 0 0 0
3 12 2 0 0 0 0
4 23 1 0 0 0 0
4 52 1 0 0 0 0
5 6 1 0 0 0 0
5 7 1 0 0 0 0
5 10 1 0 0 0 0
5 31 1 0 0 0 0
6 9 1 0 0 0 0
6 12 1 0 0 0 0
6 15 1 0 0 0 0
7 8 1 0 0 0 0
7 13 1 0 0 0 0
7 32 1 0 0 0 0
8 11 1 0 0 0 0
8 16 1 0 0 0 0
8 33 1 0 0 0 0
9 11 1 0 0 0 0
9 34 1 0 0 0 0
9 35 1 0 0 0 0
10 14 1 0 0 0 0
10 36 1 0 0 0 0
10 37 1 0 0 0 0
11 38 1 0 0 0 0
11 39 1 0 0 0 0
12 14 1 0 0 0 0
13 17 1 0 0 0 0
13 40 1 0 0 0 0
13 41 1 0 0 0 0
14 42 1 0 0 0 0
14 43 1 0 0 0 0
15 44 1 0 0 0 0
15 45 1 0 0 0 0
15 46 1 0 0 0 0
16 18 2 0 0 0 0
16 19 1 0 0 0 0
17 18 1 0 0 0 0
17 47 1 0 0 0 0
17 48 1 0 0 0 0
18 20 1 0 0 0 0
19 21 2 0 0 0 0
19 49 1 0 0 0 0
20 22 2 0 0 0 0
20 50 1 0 0 0 0
21 22 1 0 0 0 0
21 51 1 0 0 0 0
23 24 1 0 0 0 0
24 25 1 0 0 0 0
24 54 1 0 0 0 0
24 55 1 0 0 0 0
25 26 1 0 0 0 0
25 56 1 0 0 0 0
25 57 1 0 0 0 0
26 27 1 0 0 0 0
26 58 1 0 0 0 0
26 59 1 0 0 0 0
27 28 1 0 0 0 0
27 60 1 0 0 0 0
27 61 1 0 0 0 0
28 29 1 0 0 0 0
28 62 1 0 0 0 0
28 63 1 0 0 0 0
29 30 1 0 0 0 0
29 64 1 0 0 0 0
29 65 1 0 0 0 0
30 66 1 0 0 0 0
30 67 1 0 0 0 0
30 68 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
N-[(Z)-[(8R,9R,13R,14R)-3-hydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-ylidene]amino]octanamide
4.2 InChl
InChI=1S/C26H38N2O2/c1-3-4-5-6-7-8-25(30)28-27-24-14-13-23-22-11-9-18-17-19(29)10-12-20(18)21(22)15-16-26(23,24)2/h10,12,17,21-23,29H,3-9,11,13-16H2,1-2H3,(H,28,30)/b27-24-/t21-,22+,23+,26+/m0/s1
4.3 InChlKey
MISZCAAAQRDKAI-FBTWVMMMSA-N
4.4 Canonical SMILES
CCCCCCCC(=O)N/N=C\1/CC[C@H]2[C@]1(CC[C@@H]3[C@H]2CCC4=C3C=CC(=C4)O)C
4.5 lsomeric SMILES
-
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病