3. 结构
3.1 二维结构
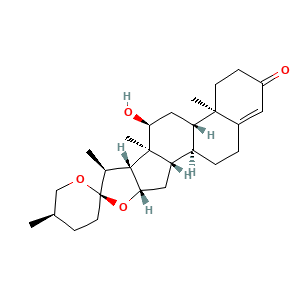
3.2 三维结构
-1
-2
-3
71 76 0 1 0 0 0 0 0999 V2000
-3.3385 -0.9555 -0.3036 O 0 0 0 0 0 0 0 0 0 0 0 0
0.4832 2.0786 1.6074 O 0 0 0 0 0 0 0 0 0 0 0 0
-4.7918 0.2972 1.1225 O 0 0 0 0 0 0 0 0 0 0 0 0
7.7551 -0.6552 1.1453 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.4584 0.8362 -0.2758 C 0 0 1 0 0 0 0 0 0 0 0 0
0.1704 -0.4962 0.2136 C 0 0 2 0 0 0 0 0 0 0 0 0
-1.8455 0.7522 0.4371 C 0 0 2 0 0 0 0 0 0 0 0 0
1.5624 -0.7587 -0.3722 C 0 0 1 0 0 0 0 0 0 0 0 0
-2.1673 -0.7565 0.4818 C 0 0 2 0 0 0 0 0 0 0 0 0
2.5113 0.4152 0.0285 C 0 0 2 0 0 0 0 0 0 0 0 0
-0.9425 -1.5103 -0.0363 C 0 0 0 0 0 0 0 0 0 0 0 0
0.4541 1.9943 0.1822 C 0 0 2 0 0 0 0 0 0 0 0 0
-3.0774 1.3724 -0.2332 C 0 0 1 0 0 0 0 0 0 0 0 0
1.8997 1.8028 -0.3256 C 0 0 0 0 0 0 0 0 0 0 0 0
3.9864 0.2154 -0.4966 C 0 0 2 0 0 0 0 0 0 0 0 0
-4.1282 0.2452 -0.1491 C 0 0 1 0 0 0 0 0 0 0 0 0
2.1365 -2.0913 0.1269 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.5983 0.8969 -1.8218 C 0 0 0 0 0 0 0 0 0 0 0 0
4.4729 -1.1934 -0.1329 C 0 0 0 0 0 0 0 0 0 0 0 0
3.5313 -2.3351 -0.4344 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.4828 2.6950 0.4071 C 0 0 0 0 0 0 0 0 0 0 0 0
4.8956 1.2828 0.1801 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.1540 0.3351 -1.2847 C 0 0 0 0 0 0 0 0 0 0 0 0
4.0812 0.3778 -2.0306 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.2343 -0.7347 -1.1424 C 0 0 0 0 0 0 0 0 0 0 0 0
6.3814 0.9805 0.0648 C 0 0 0 0 0 0 0 0 0 0 0 0
5.6748 -1.4577 0.4064 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.8501 -0.6861 0.2582 C 0 0 2 0 0 0 0 0 0 0 0 0
-5.7461 -0.7466 1.3137 C 0 0 0 0 0 0 0 0 0 0 0 0
6.6810 -0.3906 0.6092 C 0 0 0 0 0 0 0 0 0 0 0 0
-7.8622 -1.8096 0.4545 C 0 0 0 0 0 0 0 0 0 0 0 0
0.2967 -0.4473 1.3080 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.7549 1.0990 1.4744 H 0 0 0 0 0 0 0 0 0 0 0 0
1.4909 -0.8238 -1.4627 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.3879 -1.0640 1.5122 H 0 0 0 0 0 0 0 0 0 0 0 0
2.5712 0.3891 1.1288 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.0402 -1.7839 -1.0919 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.7885 -2.4358 0.5279 H 0 0 0 0 0 0 0 0 0 0 0 0
0.0580 2.9493 -0.1824 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.8862 1.5720 -1.2900 H 0 0 0 0 0 0 0 0 0 0 0 0
2.5201 2.5895 0.1183 H 0 0 0 0 0 0 0 0 0 0 0 0
1.9223 1.9588 -1.4083 H 0 0 0 0 0 0 0 0 0 0 0 0
1.4873 -2.9204 -0.1776 H 0 0 0 0 0 0 0 0 0 0 0 0
2.1721 -2.1011 1.2240 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.9385 1.8890 -2.1405 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.3126 0.1622 -2.2067 H 0 0 0 0 0 0 0 0 0 0 0 0
0.3435 0.7198 -2.3466 H 0 0 0 0 0 0 0 0 0 0 0 0
3.4711 -2.4684 -1.5216 H 0 0 0 0 0 0 0 0 0 0 0 0
3.9106 -3.2818 -0.0305 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.6664 2.5914 1.4815 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.6897 3.4390 0.2771 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.3928 3.0898 -0.0555 H 0 0 0 0 0 0 0 0 0 0 0 0
4.6460 1.3513 1.2483 H 0 0 0 0 0 0 0 0 0 0 0 0
4.7109 2.2761 -0.2458 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.6652 0.2156 -2.2586 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.6406 1.3179 -1.2694 H 0 0 0 0 0 0 0 0 0 0 0 0
3.9000 1.4129 -2.3385 H 0 0 0 0 0 0 0 0 0 0 0 0
5.0752 0.1044 -2.4034 H 0 0 0 0 0 0 0 0 0 0 0 0
3.3605 -0.2529 -2.5603 H 0 0 0 0 0 0 0 0 0 0 0 0
1.0435 2.8366 1.8459 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.0082 -0.5798 -1.9033 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.7975 -1.7240 -1.3289 H 0 0 0 0 0 0 0 0 0 0 0 0
6.9438 1.7172 0.6492 H 0 0 0 0 0 0 0 0 0 0 0 0
6.7286 1.0317 -0.9719 H 0 0 0 0 0 0 0 0 0 0 0 0
5.9786 -2.4673 0.6624 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.3705 0.2755 0.3610 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.1752 -0.6144 2.3128 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.2330 -1.7157 1.3091 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.3894 -2.7931 0.3604 H 0 0 0 0 0 0 0 0 0 0 0 0
-8.6622 -1.7458 -0.2906 H 0 0 0 0 0 0 0 0 0 0 0 0
-8.3219 -1.7487 1.4465 H 0 0 0 0 0 0 0 0 0 0 0 0
1 9 1 0 0 0 0
1 16 1 0 0 0 0
2 12 1 0 0 0 0
2 60 1 0 0 0 0
3 16 1 0 0 0 0
3 29 1 0 0 0 0
4 30 2 0 0 0 0
5 6 1 0 0 0 0
5 7 1 0 0 0 0
5 12 1 0 0 0 0
5 18 1 0 0 0 0
6 8 1 0 0 0 0
6 11 1 0 0 0 0
6 32 1 0 0 0 0
7 9 1 0 0 0 0
7 13 1 0 0 0 0
7 33 1 0 0 0 0
8 10 1 0 0 0 0
8 17 1 0 0 0 0
8 34 1 0 0 0 0
9 11 1 0 0 0 0
9 35 1 0 0 0 0
10 14 1 0 0 0 0
10 15 1 0 0 0 0
10 36 1 0 0 0 0
11 37 1 0 0 0 0
11 38 1 0 0 0 0
12 14 1 0 0 0 0
12 39 1 0 0 0 0
13 16 1 0 0 0 0
13 21 1 0 0 0 0
13 40 1 0 0 0 0
14 41 1 0 0 0 0
14 42 1 0 0 0 0
15 19 1 0 0 0 0
15 22 1 0 0 0 0
15 24 1 0 0 0 0
16 23 1 0 0 0 0
17 20 1 0 0 0 0
17 43 1 0 0 0 0
17 44 1 0 0 0 0
18 45 1 0 0 0 0
18 46 1 0 0 0 0
18 47 1 0 0 0 0
19 20 1 0 0 0 0
19 27 2 0 0 0 0
20 48 1 0 0 0 0
20 49 1 0 0 0 0
21 50 1 0 0 0 0
21 51 1 0 0 0 0
21 52 1 0 0 0 0
22 26 1 0 0 0 0
22 53 1 0 0 0 0
22 54 1 0 0 0 0
23 25 1 0 0 0 0
23 55 1 0 0 0 0
23 56 1 0 0 0 0
24 57 1 0 0 0 0
24 58 1 0 0 0 0
24 59 1 0 0 0 0
25 28 1 0 0 0 0
25 61 1 0 0 0 0
25 62 1 0 0 0 0
26 30 1 0 0 0 0
26 63 1 0 0 0 0
26 64 1 0 0 0 0
27 30 1 0 0 0 0
27 65 1 0 0 0 0
28 29 1 0 0 0 0
28 31 1 0 0 0 0
28 66 1 0 0 0 0
29 67 1 0 0 0 0
29 68 1 0 0 0 0
31 69 1 0 0 0 0
31 70 1 0 0 0 0
31 71 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
(1R,2S,4S,5'R,6R,7S,8R,9S,10S,12S,13R)-10-hydroxy-5',7,9,13-tetramethylspiro[5-oxapentacyclo[10.8.0.02,9.04,8.013,18]icos-17-ene-6,2'-oxane]-16-one
4.2 InChl
InChI=1S/C27H40O4/c1-15-7-10-27(30-14-15)16(2)24-22(31-27)12-21-19-6-5-17-11-18(28)8-9-25(17,3)20(19)13-23(29)26(21,24)4/h11,15-16,19-24,29H,5-10,12-14H2,1-4H3/t15-,16+,19-,20+,21+,22+,23+,24+,25+,26-,27-/m1/s1
4.3 InChlKey
FTBCSMASEBPQAV-NGJIIWHQSA-N
4.4 Canonical SMILES
CC1CCC2(C(C3C(O2)CC4C3(C(CC5C4CCC6=CC(=O)CCC56C)O)C)C)OC1
4.5 lsomeric SMILES
C[C@@H]1CC[C@@]2([C@H]([C@H]3[C@@H](O2)C[C@@H]4[C@@]3([C@H](C[C@H]5[C@H]4CCC6=CC(=O)CC[C@]56C)O)C)C)OC1
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病