3. 结构
3.1 二维结构
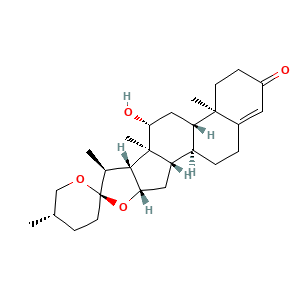
3.2 三维结构
-1
-2
-3
71 76 0 1 0 0 0 0 0999 V2000
-3.2773 1.1709 0.3288 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.1484 -3.1258 -0.0934 O 0 0 0 0 0 0 0 0 0 0 0 0
-4.7870 0.0766 -1.1635 O 0 0 0 0 0 0 0 0 0 0 0 0
7.8007 0.5858 -1.1472 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.4685 -0.7265 0.1716 C 0 0 1 0 0 0 0 0 0 0 0 0
0.2076 0.6123 -0.2232 C 0 0 2 0 0 0 0 0 0 0 0 0
-1.8557 -0.5498 -0.5229 C 0 0 2 0 0 0 0 0 0 0 0 0
1.6082 0.7797 0.3752 C 0 0 1 0 0 0 0 0 0 0 0 0
-2.1167 0.9717 -0.4721 C 0 0 2 0 0 0 0 0 0 0 0 0
2.5139 -0.3860 -0.1327 C 0 0 2 0 0 0 0 0 0 0 0 0
-0.8670 1.6453 0.0945 C 0 0 0 0 0 0 0 0 0 0 0 0
0.3998 -1.8536 -0.4214 C 0 0 1 0 0 0 0 0 0 0 0 0
-3.1150 -1.1575 0.1086 C 0 0 1 0 0 0 0 0 0 0 0 0
1.8533 -1.7734 0.0877 C 0 0 0 0 0 0 0 0 0 0 0 0
3.9942 -0.2882 0.4030 C 0 0 2 0 0 0 0 0 0 0 0 0
-4.1170 0.0174 0.1048 C 0 0 1 0 0 0 0 0 0 0 0 0
2.2277 2.1292 -0.0101 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.6038 -0.9034 1.7089 C 0 0 0 0 0 0 0 0 0 0 0 0
4.5322 1.1287 0.1643 C 0 0 0 0 0 0 0 0 0 0 0 0
3.6315 2.2735 0.5642 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.5919 -2.4102 -0.6188 C 0 0 0 0 0 0 0 0 0 0 0 0
4.8634 -1.3235 -0.3689 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.1367 -0.1008 1.2441 C 0 0 0 0 0 0 0 0 0 0 0 0
4.0823 -0.5897 1.9162 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.1973 0.9964 1.1633 C 0 0 0 0 0 0 0 0 0 0 0 0
6.3594 -1.0898 -0.2284 C 0 0 0 0 0 0 0 0 0 0 0 0
5.7453 1.3947 -0.3485 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.8102 1.0948 -0.2373 C 0 0 1 0 0 0 0 0 0 0 0 0
-5.6946 1.1713 -1.2806 C 0 0 0 0 0 0 0 0 0 0 0 0
6.7130 0.3135 -0.6430 C 0 0 0 0 0 0 0 0 0 0 0 0
-7.7454 -0.0839 -0.5230 C 0 0 0 0 0 0 0 0 0 0 0 0
0.3326 0.6277 -1.3195 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.7681 -0.8410 -1.5787 H 0 0 0 0 0 0 0 0 0 0 0 0
1.5418 0.7522 1.4676 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.3243 1.3518 -1.4807 H 0 0 0 0 0 0 0 0 0 0 0 0
2.5729 -0.2628 -1.2267 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.9566 1.8506 1.1661 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.6794 2.6006 -0.4064 H 0 0 0 0 0 0 0 0 0 0 0 0
0.4069 -1.7805 -1.5164 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.9315 -1.4366 1.1486 H 0 0 0 0 0 0 0 0 0 0 0 0
2.4294 -2.5504 -0.4286 H 0 0 0 0 0 0 0 0 0 0 0 0
1.8825 -2.0562 1.1454 H 0 0 0 0 0 0 0 0 0 0 0 0
1.6088 2.9512 0.3678 H 0 0 0 0 0 0 0 0 0 0 0 0
2.2620 2.2323 -1.1024 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.0119 -1.8879 1.9624 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.2611 -0.1516 2.1571 H 0 0 0 0 0 0 0 0 0 0 0 0
0.3536 -0.8337 2.2311 H 0 0 0 0 0 0 0 0 0 0 0 0
3.5767 2.3159 1.6591 H 0 0 0 0 0 0 0 0 0 0 0 0
4.0444 3.2370 0.2408 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.7560 -2.2268 -1.6857 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.8506 -3.2105 -0.5315 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.5300 -2.7769 -0.1905 H 0 0 0 0 0 0 0 0 0 0 0 0
4.6138 -1.2849 -1.4387 H 0 0 0 0 0 0 0 0 0 0 0 0
4.6395 -2.3437 -0.0350 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.6351 -0.0327 2.2169 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.6374 -1.0747 1.1997 H 0 0 0 0 0 0 0 0 0 0 0 0
3.8612 -1.6402 2.1319 H 0 0 0 0 0 0 0 0 0 0 0 0
5.0861 -0.3889 2.3087 H 0 0 0 0 0 0 0 0 0 0 0 0
3.3867 0.0189 2.5022 H 0 0 0 0 0 0 0 0 0 0 0 0
0.4237 -3.8039 -0.4912 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.9752 0.8201 1.9153 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.7326 1.9584 1.4152 H 0 0 0 0 0 0 0 0 0 0 0 0
6.8945 -1.7899 -0.8795 H 0 0 0 0 0 0 0 0 0 0 0 0
6.7020 -1.2504 0.7986 H 0 0 0 0 0 0 0 0 0 0 0 0
6.0868 2.4111 -0.5143 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.4092 2.0121 -0.2941 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.1149 1.1456 -2.2917 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.1404 2.1131 -1.1910 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.2149 -1.0411 -0.5373 H 0 0 0 0 0 0 0 0 0 0 0 0
-8.2296 0.0405 -1.4977 H 0 0 0 0 0 0 0 0 0 0 0 0
-8.5343 -0.1444 0.2341 H 0 0 0 0 0 0 0 0 0 0 0 0
1 9 1 0 0 0 0
1 16 1 0 0 0 0
2 12 1 0 0 0 0
2 60 1 0 0 0 0
3 16 1 0 0 0 0
3 29 1 0 0 0 0
4 30 2 0 0 0 0
5 6 1 0 0 0 0
5 7 1 0 0 0 0
5 12 1 0 0 0 0
5 18 1 0 0 0 0
6 8 1 0 0 0 0
6 11 1 0 0 0 0
6 32 1 0 0 0 0
7 9 1 0 0 0 0
7 13 1 0 0 0 0
7 33 1 0 0 0 0
8 10 1 0 0 0 0
8 17 1 0 0 0 0
8 34 1 0 0 0 0
9 11 1 0 0 0 0
9 35 1 0 0 0 0
10 14 1 0 0 0 0
10 15 1 0 0 0 0
10 36 1 0 0 0 0
11 37 1 0 0 0 0
11 38 1 0 0 0 0
12 14 1 0 0 0 0
12 39 1 0 0 0 0
13 16 1 0 0 0 0
13 21 1 0 0 0 0
13 40 1 0 0 0 0
14 41 1 0 0 0 0
14 42 1 0 0 0 0
15 19 1 0 0 0 0
15 22 1 0 0 0 0
15 24 1 0 0 0 0
16 23 1 0 0 0 0
17 20 1 0 0 0 0
17 43 1 0 0 0 0
17 44 1 0 0 0 0
18 45 1 0 0 0 0
18 46 1 0 0 0 0
18 47 1 0 0 0 0
19 20 1 0 0 0 0
19 27 2 0 0 0 0
20 48 1 0 0 0 0
20 49 1 0 0 0 0
21 50 1 0 0 0 0
21 51 1 0 0 0 0
21 52 1 0 0 0 0
22 26 1 0 0 0 0
22 53 1 0 0 0 0
22 54 1 0 0 0 0
23 25 1 0 0 0 0
23 55 1 0 0 0 0
23 56 1 0 0 0 0
24 57 1 0 0 0 0
24 58 1 0 0 0 0
24 59 1 0 0 0 0
25 28 1 0 0 0 0
25 61 1 0 0 0 0
25 62 1 0 0 0 0
26 30 1 0 0 0 0
26 63 1 0 0 0 0
26 64 1 0 0 0 0
27 30 1 0 0 0 0
27 65 1 0 0 0 0
28 29 1 0 0 0 0
28 31 1 0 0 0 0
28 66 1 0 0 0 0
29 67 1 0 0 0 0
29 68 1 0 0 0 0
31 69 1 0 0 0 0
31 70 1 0 0 0 0
31 71 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
(1R,2S,4S,5'S,6R,7S,8R,9S,10R,12S,13R)-10-hydroxy-5',7,9,13-tetramethylspiro[5-oxapentacyclo[10.8.0.02,9.04,8.013,18]icos-17-ene-6,2'-oxane]-16-one
4.2 InChl
InChI=1S/C27H40O4/c1-15-7-10-27(30-14-15)16(2)24-22(31-27)12-21-19-6-5-17-11-18(28)8-9-25(17,3)20(19)13-23(29)26(21,24)4/h11,15-16,19-24,29H,5-10,12-14H2,1-4H3/t15-,16-,19+,20-,21-,22-,23+,24-,25-,26+,27+/m0/s1
4.3 InChlKey
FTBCSMASEBPQAV-HFHIOTRISA-N
4.4 Canonical SMILES
CC1CCC2(C(C3C(O2)CC4C3(C(CC5C4CCC6=CC(=O)CCC56C)O)C)C)OC1
4.5 lsomeric SMILES
C[C@H]1CC[C@@]2([C@H]([C@H]3[C@@H](O2)C[C@@H]4[C@@]3([C@@H](C[C@H]5[C@H]4CCC6=CC(=O)CC[C@]56C)O)C)C)OC1
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病