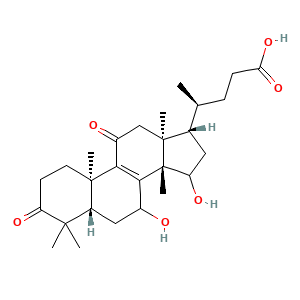
3. 结构
3.1 二维结构
3.2 三维结构
-1
-2
-3
73 76 0 1 0 0 0 0 0999 V2000
1.4223 -3.1311 0.2801 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.0359 -2.6106 -1.5580 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.9719 2.9857 -0.0998 O 0 0 0 0 0 0 0 0 0 0 0 0
-6.5062 0.6198 1.9434 O 0 0 0 0 0 0 0 0 0 0 0 0
7.1687 -0.4306 2.4555 O 0 0 0 0 0 0 0 0 0 0 0 0
8.1078 -0.0457 0.4238 O 0 0 0 0 0 0 0 0 0 0 0 0
0.7464 -0.7521 0.0673 C 0 0 2 0 0 0 0 0 0 0 0 0
1.5126 0.4268 -0.6136 C 0 0 2 0 0 0 0 0 0 0 0 0
2.9881 0.0777 -0.2916 C 0 0 1 0 0 0 0 0 0 0 0 0
1.5770 -1.9448 -0.4811 C 0 0 1 0 0 0 0 0 0 0 0 0
-0.7531 -0.6135 -0.2287 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.9118 0.7723 -0.2802 C 0 0 1 0 0 0 0 0 0 0 0 0
3.0438 -1.4591 -0.4395 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3619 0.6057 -0.1941 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.5864 -0.5785 0.1904 C 0 0 2 0 0 0 0 0 0 0 0 0
1.0278 1.7371 -0.0130 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.5325 -1.9014 -0.4242 C 0 0 1 0 0 0 0 0 0 0 0 0
4.0592 0.7890 -1.1100 C 0 0 2 0 0 0 0 0 0 0 0 0
-3.0363 -1.7389 -0.6399 C 0 0 0 0 0 0 0 0 0 0 0 0
0.8320 -0.7784 1.6299 C 0 0 0 0 0 0 0 0 0 0 0 0
1.3089 0.4973 -2.1615 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.4867 1.8541 -0.1000 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.1466 -0.6099 0.3496 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.4651 1.8720 0.6709 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.1975 1.1638 -1.7466 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.9843 1.9566 0.7098 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.6017 0.6435 1.1064 C 0 0 0 0 0 0 0 0 0 0 0 0
5.4590 0.2993 -0.6886 C 0 0 0 0 0 0 0 0 0 0 0 0
3.9085 2.3059 -0.9626 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.5494 -1.8392 1.2071 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.9409 -0.6962 -0.9616 C 0 0 0 0 0 0 0 0 0 0 0 0
5.7686 0.5230 0.7930 C 0 0 0 0 0 0 0 0 0 0 0 0
7.1376 0.0008 1.1672 C 0 0 0 0 0 0 0 0 0 0 0 0
3.1406 0.3255 0.7646 H 0 0 0 0 0 0 0 0 0 0 0 0
1.3136 -2.1807 -1.5167 H 0 0 0 0 0 0 0 0 0 0 0 0
3.5388 -1.7683 -1.3675 H 0 0 0 0 0 0 0 0 0 0 0 0
3.6007 -1.9118 0.3889 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.2110 -0.7363 1.2170 H 0 0 0 0 0 0 0 0 0 0 0 0
1.4128 2.6038 -0.5533 H 0 0 0 0 0 0 0 0 0 0 0 0
1.3193 1.8549 1.0358 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.3731 -2.5316 0.4594 H 0 0 0 0 0 0 0 0 0 0 0 0
3.9602 0.5444 -2.1748 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.2626 -1.6078 -1.7045 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.5077 -2.6908 -0.3738 H 0 0 0 0 0 0 0 0 0 0 0 0
0.4025 0.1163 2.0930 H 0 0 0 0 0 0 0 0 0 0 0 0
1.8563 -0.8749 2.0018 H 0 0 0 0 0 0 0 0 0 0 0 0
0.2742 -1.6314 2.0368 H 0 0 0 0 0 0 0 0 0 0 0 0
1.7762 -0.3434 -2.6840 H 0 0 0 0 0 0 0 0 0 0 0 0
1.7308 1.4101 -2.5912 H 0 0 0 0 0 0 0 0 0 0 0 0
0.2526 0.4922 -2.4473 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.1275 2.8681 0.3751 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.0878 1.6967 1.6876 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.2523 1.3447 -1.9454 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.8511 0.4051 -2.4558 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.6817 2.0952 -2.0113 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.4029 2.2670 -0.2518 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.2787 2.7125 1.4474 H 0 0 0 0 0 0 0 0 0 0 0 0
5.5856 -0.7626 -0.9317 H 0 0 0 0 0 0 0 0 0 0 0 0
6.2109 0.8227 -1.2943 H 0 0 0 0 0 0 0 0 0 0 0 0
2.0290 -3.7957 -0.0883 H 0 0 0 0 0 0 0 0 0 0 0 0
3.7019 2.6098 0.0684 H 0 0 0 0 0 0 0 0 0 0 0 0
3.1487 2.7150 -1.6298 H 0 0 0 0 0 0 0 0 0 0 0 0
4.8407 2.8029 -1.2578 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.3350 -2.7851 0.7015 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.6239 -1.8409 1.4278 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.0259 -1.8397 2.1702 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.6225 -1.5423 -1.5781 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.8755 0.2111 -1.5618 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.0101 -0.8328 -0.7553 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.4876 -3.4713 -1.5882 H 0 0 0 0 0 0 0 0 0 0 0 0
5.7365 1.5854 1.0535 H 0 0 0 0 0 0 0 0 0 0 0 0
5.0513 -0.0128 1.4209 H 0 0 0 0 0 0 0 0 0 0 0 0
8.0531 -0.7743 2.7044 H 0 0 0 0 0 0 0 0 0 0 0 0
1 10 1 0 0 0 0
1 60 1 0 0 0 0
2 17 1 0 0 0 0
2 70 1 0 0 0 0
3 22 2 0 0 0 0
4 27 2 0 0 0 0
5 33 1 0 0 0 0
5 73 1 0 0 0 0
6 33 2 0 0 0 0
7 8 1 0 0 0 0
7 10 1 0 0 0 0
7 11 1 0 0 0 0
7 20 1 0 0 0 0
8 9 1 0 0 0 0
8 16 1 0 0 0 0
8 21 1 0 0 0 0
9 13 1 0 0 0 0
9 18 1 0 0 0 0
9 34 1 0 0 0 0
10 13 1 0 0 0 0
10 35 1 0 0 0 0
11 14 2 0 0 0 0
11 17 1 0 0 0 0
12 14 1 0 0 0 0
12 15 1 0 0 0 0
12 24 1 0 0 0 0
12 25 1 0 0 0 0
13 36 1 0 0 0 0
13 37 1 0 0 0 0
14 22 1 0 0 0 0
15 19 1 0 0 0 0
15 23 1 0 0 0 0
15 38 1 0 0 0 0
16 22 1 0 0 0 0
16 39 1 0 0 0 0
16 40 1 0 0 0 0
17 19 1 0 0 0 0
17 41 1 0 0 0 0
18 28 1 0 0 0 0
18 29 1 0 0 0 0
18 42 1 0 0 0 0
19 43 1 0 0 0 0
19 44 1 0 0 0 0
20 45 1 0 0 0 0
20 46 1 0 0 0 0
20 47 1 0 0 0 0
21 48 1 0 0 0 0
21 49 1 0 0 0 0
21 50 1 0 0 0 0
23 27 1 0 0 0 0
23 30 1 0 0 0 0
23 31 1 0 0 0 0
24 26 1 0 0 0 0
24 51 1 0 0 0 0
24 52 1 0 0 0 0
25 53 1 0 0 0 0
25 54 1 0 0 0 0
25 55 1 0 0 0 0
26 27 1 0 0 0 0
26 56 1 0 0 0 0
26 57 1 0 0 0 0
28 32 1 0 0 0 0
28 58 1 0 0 0 0
28 59 1 0 0 0 0
29 61 1 0 0 0 0
29 62 1 0 0 0 0
29 63 1 0 0 0 0
30 64 1 0 0 0 0
30 65 1 0 0 0 0
30 66 1 0 0 0 0
31 67 1 0 0 0 0
31 68 1 0 0 0 0
31 69 1 0 0 0 0
32 33 1 0 0 0 0
32 71 1 0 0 0 0
32 72 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
(4S)-4-[(5S,10R,13S,14S,17S)-7,15-dihydroxy-4,4,10,13,14-pentamethyl-3,11-dioxo-2,5,6,7,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid
4.2 InChl
InChI=1S/C27H40O6/c1-14(7-8-21(32)33)15-11-20(31)27(6)23-16(28)12-18-24(2,3)19(30)9-10-25(18,4)22(23)17(29)13-26(15,27)5/h14-16,18,20,28,31H,7-13H2,1-6H3,(H,32,33)/t14-,15-,16?,18+,20?,25+,26-,27+/m0/s1
4.3 InChlKey
JBKAGLHPUJBTTD-OGMYJHHNSA-N
4.4 Canonical SMILES
CC(CCC(=O)O)C1CC(C2(C1(CC(=O)C3=C2C(CC4C3(CCC(=O)C4(C)C)C)O)C)C)O
4.5 lsomeric SMILES
C[C@@H](CCC(=O)O)[C@@H]1CC([C@]2([C@]1(CC(=O)C3=C2C(C[C@H]4[C@]3(CCC(=O)C4(C)C)C)O)C)C)O
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病