3. 结构
3.1 二维结构
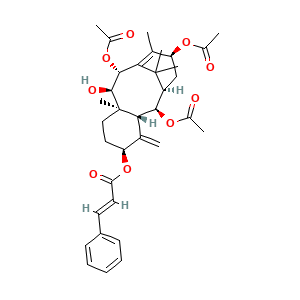
3.2 三维结构
-1
-2
-3
88 91 0 1 0 0 0 0 0999 V2000
3.3751 2.0895 -0.5974 O 0 0 0 0 0 0 0 0 0 0 0 0
1.8196 -2.0927 3.3433 O 0 0 0 0 0 0 0 0 0 0 0 0
1.7338 -3.6244 1.0428 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.8630 0.7476 -1.8688 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.9988 2.3833 0.9542 O 0 0 0 0 0 0 0 0 0 0 0 0
5.3279 1.1485 -1.3957 O 0 0 0 0 0 0 0 0 0 0 0 0
0.0459 -4.2942 -0.3852 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.6331 1.5584 -4.0197 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.7499 3.2002 2.2208 O 0 0 0 0 0 0 0 0 0 0 0 0
1.6715 0.0947 2.2421 C 0 0 2 0 0 0 0 0 0 0 0 0
1.7031 1.0363 0.9416 C 0 0 2 0 0 0 0 0 0 0 0 0
2.6604 -0.0477 -1.3590 C 0 0 2 0 0 0 0 0 0 0 0 0
2.5354 -1.5696 -1.1082 C 0 0 0 0 0 0 0 0 0 0 0 0
2.9026 0.8418 -0.0661 C 0 0 1 0 0 0 0 0 0 0 0 0
2.0038 -1.4418 2.0633 C 0 0 2 0 0 0 0 0 0 0 0 0
1.2066 -1.7446 -0.3520 C 0 0 0 0 0 0 0 0 0 0 0 0
0.2436 0.2215 2.8833 C 0 0 0 0 0 0 0 0 0 0 0 0
1.2070 -2.2943 1.0507 C 0 0 1 0 0 0 0 0 0 0 0 0
1.4845 0.4521 -2.2483 C 0 0 0 0 0 0 0 0 0 0 0 0
1.3671 2.4665 1.4246 C 0 0 0 0 0 0 0 0 0 0 0 0
0.1295 -0.2691 -2.0619 C 0 0 1 0 0 0 0 0 0 0 0 0
-0.2318 1.6562 3.1584 C 0 0 0 0 0 0 0 0 0 0 0 0
2.7228 0.6132 3.2786 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.0352 2.6112 1.9791 C 0 0 2 0 0 0 0 0 0 0 0 0
0.0654 -1.2554 -0.9072 C 0 0 0 0 0 0 0 0 0 0 0 0
3.8561 -2.1107 -0.4972 C 0 0 0 0 0 0 0 0 0 0 0 0
2.4488 -2.3737 -2.4471 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3275 -1.5610 -0.4148 C 0 0 0 0 0 0 0 0 0 0 0 0
2.2070 3.5120 1.5194 C 0 0 0 0 0 0 0 0 0 0 0 0
4.4449 1.9947 -1.4322 C 0 0 0 0 0 0 0 0 0 0 0 0
1.0483 -4.5218 0.2786 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.9158 1.7039 -2.8382 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.3031 2.4869 1.3364 C 0 0 0 0 0 0 0 0 0 0 0 0
4.3890 3.1066 -2.4353 C 0 0 0 0 0 0 0 0 0 0 0 0
1.7106 -5.8634 0.3653 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3823 2.9963 -2.2398 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.0987 1.5728 0.4858 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.2213 1.0260 0.9737 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.1014 0.1123 0.2239 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.0367 0.0916 -1.1600 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.9764 -0.7083 0.9172 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.8698 -0.7730 -1.8699 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.8094 -1.5729 0.2072 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.7562 -1.6052 -1.1864 C 0 0 0 0 0 0 0 0 0 0 0 0
0.8189 0.7770 0.3608 H 0 0 0 0 0 0 0 0 0 0 0 0
3.5187 0.0222 -2.0311 H 0 0 0 0 0 0 0 0 0 0 0 0
3.7374 0.4388 0.5134 H 0 0 0 0 0 0 0 0 0 0 0 0
3.0693 -1.5526 1.8791 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.4882 -0.2498 2.2178 H 0 0 0 0 0 0 0 0 0 0 0 0
0.1946 -0.3298 3.8301 H 0 0 0 0 0 0 0 0 0 0 0 0
0.1991 -2.3799 1.4691 H 0 0 0 0 0 0 0 0 0 0 0 0
1.4060 1.5382 -2.1264 H 0 0 0 0 0 0 0 0 0 0 0 0
1.7805 0.3117 -3.2982 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.1448 -0.8100 -2.9769 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.2737 1.6089 3.4904 H 0 0 0 0 0 0 0 0 0 0 0 0
0.3333 2.0430 4.0167 H 0 0 0 0 0 0 0 0 0 0 0 0
2.6605 0.0726 4.2288 H 0 0 0 0 0 0 0 0 0 0 0 0
2.5949 1.6660 3.5402 H 0 0 0 0 0 0 0 0 0 0 0 0
3.7414 0.4955 2.8933 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.1886 3.6371 2.3408 H 0 0 0 0 0 0 0 0 0 0 0 0
3.7808 -3.1645 -0.2144 H 0 0 0 0 0 0 0 0 0 0 0 0
4.2285 -1.5537 0.3585 H 0 0 0 0 0 0 0 0 0 0 0 0
4.6677 -2.0484 -1.2348 H 0 0 0 0 0 0 0 0 0 0 0 0
2.4560 -3.4531 -2.2500 H 0 0 0 0 0 0 0 0 0 0 0 0
3.3071 -2.1523 -3.0933 H 0 0 0 0 0 0 0 0 0 0 0 0
1.5555 -2.1805 -3.0431 H 0 0 0 0 0 0 0 0 0 0 0 0
2.6481 -2.0044 3.8441 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.4092 -2.4768 0.1711 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.0086 -1.6850 -1.2640 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.6913 -0.7440 0.2080 H 0 0 0 0 0 0 0 0 0 0 0 0
3.2552 3.4544 1.2586 H 0 0 0 0 0 0 0 0 0 0 0 0
1.8741 4.4642 1.9213 H 0 0 0 0 0 0 0 0 0 0 0 0
4.4112 4.0711 -1.9218 H 0 0 0 0 0 0 0 0 0 0 0 0
3.4851 3.0141 -3.0425 H 0 0 0 0 0 0 0 0 0 0 0 0
5.2596 3.0439 -3.0944 H 0 0 0 0 0 0 0 0 0 0 0 0
1.7145 -6.2089 1.4021 H 0 0 0 0 0 0 0 0 0 0 0 0
2.7300 -5.7985 -0.0235 H 0 0 0 0 0 0 0 0 0 0 0 0
1.1518 -6.5833 -0.2395 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.2434 3.7918 -2.9793 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.7732 3.2561 -1.3736 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.4439 2.9468 -1.9967 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.7190 1.3333 -0.4907 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.5403 1.2573 1.9877 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.3777 0.7354 -1.7339 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.0284 -0.6955 2.0026 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.8319 -0.7954 -2.9552 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.4995 -2.2213 0.7392 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.4055 -2.2777 -1.7391 H 0 0 0 0 0 0 0 0 0 0 0 0
1 14 1 0 0 0 0
1 30 1 0 0 0 0
2 15 1 0 0 0 0
2 67 1 0 0 0 0
3 18 1 0 0 0 0
3 31 1 0 0 0 0
4 21 1 0 0 0 0
4 32 1 0 0 0 0
5 24 1 0 0 0 0
5 33 1 0 0 0 0
6 30 2 0 0 0 0
7 31 2 0 0 0 0
8 32 2 0 0 0 0
9 33 2 0 0 0 0
10 11 1 0 0 0 0
10 15 1 0 0 0 0
10 17 1 0 0 0 0
10 23 1 0 0 0 0
11 14 1 0 0 0 0
11 20 1 0 0 0 0
11 45 1 0 0 0 0
12 13 1 0 0 0 0
12 14 1 0 0 0 0
12 19 1 0 0 0 0
12 46 1 0 0 0 0
13 16 1 0 0 0 0
13 26 1 0 0 0 0
13 27 1 0 0 0 0
14 47 1 0 0 0 0
15 18 1 0 0 0 0
15 48 1 0 0 0 0
16 18 1 0 0 0 0
16 25 2 0 0 0 0
17 22 1 0 0 0 0
17 49 1 0 0 0 0
17 50 1 0 0 0 0
18 51 1 0 0 0 0
19 21 1 0 0 0 0
19 52 1 0 0 0 0
19 53 1 0 0 0 0
20 24 1 0 0 0 0
20 29 2 0 0 0 0
21 25 1 0 0 0 0
21 54 1 0 0 0 0
22 24 1 0 0 0 0
22 55 1 0 0 0 0
22 56 1 0 0 0 0
23 57 1 0 0 0 0
23 58 1 0 0 0 0
23 59 1 0 0 0 0
24 60 1 0 0 0 0
25 28 1 0 0 0 0
26 61 1 0 0 0 0
26 62 1 0 0 0 0
26 63 1 0 0 0 0
27 64 1 0 0 0 0
27 65 1 0 0 0 0
27 66 1 0 0 0 0
28 68 1 0 0 0 0
28 69 1 0 0 0 0
28 70 1 0 0 0 0
29 71 1 0 0 0 0
29 72 1 0 0 0 0
30 34 1 0 0 0 0
31 35 1 0 0 0 0
32 36 1 0 0 0 0
33 37 1 0 0 0 0
34 73 1 0 0 0 0
34 74 1 0 0 0 0
34 75 1 0 0 0 0
35 76 1 0 0 0 0
35 77 1 0 0 0 0
35 78 1 0 0 0 0
36 79 1 0 0 0 0
36 80 1 0 0 0 0
36 81 1 0 0 0 0
37 38 2 0 0 0 0
37 82 1 0 0 0 0
38 39 1 0 0 0 0
38 83 1 0 0 0 0
39 40 2 0 0 0 0
39 41 1 0 0 0 0
40 42 1 0 0 0 0
40 84 1 0 0 0 0
41 43 2 0 0 0 0
41 85 1 0 0 0 0
42 44 2 0 0 0 0
42 86 1 0 0 0 0
43 44 1 0 0 0 0
43 87 1 0 0 0 0
44 88 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
[(1R,2R,3R,5S,8R,9R,10R,13S)-2,10,13-triacetyloxy-9-hydroxy-8,12,15,15-tetramethyl-4-methylidene-5-tricyclo[9.3.1.03,8]pentadec-11-enyl] (E)-3-phenylprop-2-enoate
4.2 InChl
InChI=1S/C35H44O9/c1-19-26(44-28(39)15-14-24-12-10-9-11-13-24)16-17-35(8)30(19)31(42-22(4)37)25-18-27(41-21(3)36)20(2)29(34(25,6)7)32(33(35)40)43-23(5)38/h9-15,25-27,30-33,40H,1,16-18H2,2-8H3/b15-14+/t25-,26-,27-,30-,31+,32+,33-,35+/m0/s1
4.3 InChlKey
MNKBCBWUAFDXSP-JVJUJCKXSA-N
4.4 Canonical SMILES
CC1=C2C(C(C3(CCC(C(=C)C3C(C(C2(C)C)CC1OC(=O)C)OC(=O)C)OC(=O)C=CC4=CC=CC=C4)C)O)OC(=O)C
4.5 lsomeric SMILES
CC1=C2[C@H]([C@@H]([C@@]3(CC[C@@H](C(=C)[C@H]3[C@@H]([C@@H](C2(C)C)C[C@@H]1OC(=O)C)OC(=O)C)OC(=O)/C=C/C4=CC=CC=C4)C)O)OC(=O)C
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病