3. 结构
3.1 二维结构
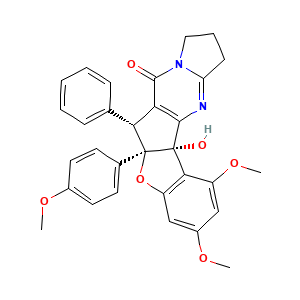
3.2 三维结构
-1
-2
-3
67 73 0 1 0 0 0 0 0999 V2000
-0.0181 -1.8960 -0.9401 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.5932 -0.6822 2.1691 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.1374 3.3877 -1.9579 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.7888 -0.5565 1.5302 O 0 0 0 0 0 0 0 0 0 0 0 0
-4.2372 -4.0864 -1.6604 O 0 0 0 0 0 0 0 0 0 0 0 0
5.2883 -1.7278 2.4993 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.4387 3.6347 -0.0464 N 0 0 0 0 0 0 0 0 0 0 0 0
-1.9108 1.7991 1.4071 N 0 0 0 0 0 0 0 0 0 0 0 0
0.3670 -0.7157 -0.1591 C 0 0 2 0 0 0 0 0 0 0 0 0
-0.8390 -0.3778 0.8074 C 0 0 2 0 0 0 0 0 0 0 0 0
0.4058 0.4966 -1.1841 C 0 0 1 0 0 0 0 0 0 0 0 0
-1.0682 1.0858 0.5915 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.3997 1.5474 -0.4783 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.8948 -1.2707 0.2530 C 0 0 0 0 0 0 0 0 0 0 0 0
1.6949 -0.9890 0.5586 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3499 -2.1034 -0.7080 C 0 0 0 0 0 0 0 0 0 0 0 0
1.7844 0.8740 -1.6946 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.6197 2.9447 -0.9224 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.0477 3.0361 1.0566 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.2348 -1.3698 0.5938 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.8431 5.0154 -0.1812 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.9116 4.0488 1.7437 C 0 0 0 0 0 0 0 0 0 0 0 0
2.2755 0.0008 1.3511 C 0 0 0 0 0 0 0 0 0 0 0 0
2.3211 -2.2271 0.4175 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.3612 5.3667 1.2034 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.1052 -3.0584 -1.3629 C 0 0 0 0 0 0 0 0 0 0 0 0
2.5441 1.8202 -1.0094 C 0 0 0 0 0 0 0 0 0 0 0 0
2.2866 0.2738 -2.8476 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.0192 -2.3233 -0.0596 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.4569 -3.1635 -1.0326 C 0 0 0 0 0 0 0 0 0 0 0 0
3.4835 -0.2478 2.0029 C 0 0 0 0 0 0 0 0 0 0 0 0
3.5290 -2.4756 1.0695 C 0 0 0 0 0 0 0 0 0 0 0 0
3.8109 2.1674 -1.4789 C 0 0 0 0 0 0 0 0 0 0 0 0
3.5534 0.6209 -3.3170 C 0 0 0 0 0 0 0 0 0 0 0 0
4.1102 -1.4859 1.8621 C 0 0 0 0 0 0 0 0 0 0 0 0
4.3155 1.5678 -2.6326 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.7627 -1.0082 2.8850 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.6073 -4.9083 -2.6411 C 0 0 0 0 0 0 0 0 0 0 0 0
5.8753 -3.0147 2.3125 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.1880 0.2332 -2.0737 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.2051 0.0960 2.6032 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.6349 5.0634 -0.9366 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.0047 5.6391 -0.5046 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.9510 3.8905 1.4391 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.8336 3.9727 2.8308 H 0 0 0 0 0 0 0 0 0 0 0 0
1.7835 0.9513 1.5281 H 0 0 0 0 0 0 0 0 0 0 0 0
1.9012 -3.0227 -0.1902 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.1082 6.1650 1.1866 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.5277 5.6980 1.8356 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.6050 -3.6726 -2.1017 H 0 0 0 0 0 0 0 0 0 0 0 0
2.1711 2.3408 -0.1335 H 0 0 0 0 0 0 0 0 0 0 0 0
1.7037 -0.4675 -3.3876 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.0745 -2.4164 0.1865 H 0 0 0 0 0 0 0 0 0 0 0 0
3.9249 0.5226 2.6291 H 0 0 0 0 0 0 0 0 0 0 0 0
3.9620 -3.4583 0.9172 H 0 0 0 0 0 0 0 0 0 0 0 0
4.4006 2.9114 -0.9514 H 0 0 0 0 0 0 0 0 0 0 0 0
3.9467 0.1536 -4.2151 H 0 0 0 0 0 0 0 0 0 0 0 0
5.3011 1.8395 -2.9992 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.6674 -0.6462 3.3815 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.8954 -0.5848 3.3974 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.7402 -2.1012 2.9536 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.8269 -5.5367 -2.1987 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.2350 -4.3162 -3.4840 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.3732 -5.5826 -3.0383 H 0 0 0 0 0 0 0 0 0 0 0 0
6.1459 -3.1853 1.2650 H 0 0 0 0 0 0 0 0 0 0 0 0
5.2362 -3.8101 2.7109 H 0 0 0 0 0 0 0 0 0 0 0 0
6.8054 -3.0327 2.8899 H 0 0 0 0 0 0 0 0 0 0 0 0
1 9 1 0 0 0 0
1 16 1 0 0 0 0
2 10 1 0 0 0 0
2 41 1 0 0 0 0
3 18 2 0 0 0 0
4 20 1 0 0 0 0
4 37 1 0 0 0 0
5 30 1 0 0 0 0
5 38 1 0 0 0 0
6 35 1 0 0 0 0
6 39 1 0 0 0 0
7 18 1 0 0 0 0
7 19 1 0 0 0 0
7 21 1 0 0 0 0
8 12 1 0 0 0 0
8 19 2 0 0 0 0
9 10 1 0 0 0 0
9 11 1 0 0 0 0
9 15 1 0 0 0 0
10 12 1 0 0 0 0
10 14 1 0 0 0 0
11 13 1 0 0 0 0
11 17 1 0 0 0 0
11 40 1 0 0 0 0
12 13 2 0 0 0 0
13 18 1 0 0 0 0
14 16 2 0 0 0 0
14 20 1 0 0 0 0
15 23 2 0 0 0 0
15 24 1 0 0 0 0
16 26 1 0 0 0 0
17 27 2 0 0 0 0
17 28 1 0 0 0 0
19 22 1 0 0 0 0
20 29 2 0 0 0 0
21 25 1 0 0 0 0
21 42 1 0 0 0 0
21 43 1 0 0 0 0
22 25 1 0 0 0 0
22 44 1 0 0 0 0
22 45 1 0 0 0 0
23 31 1 0 0 0 0
23 46 1 0 0 0 0
24 32 2 0 0 0 0
24 47 1 0 0 0 0
25 48 1 0 0 0 0
25 49 1 0 0 0 0
26 30 2 0 0 0 0
26 50 1 0 0 0 0
27 33 1 0 0 0 0
27 51 1 0 0 0 0
28 34 2 0 0 0 0
28 52 1 0 0 0 0
29 30 1 0 0 0 0
29 53 1 0 0 0 0
31 35 2 0 0 0 0
31 54 1 0 0 0 0
32 35 1 0 0 0 0
32 55 1 0 0 0 0
33 36 2 0 0 0 0
33 56 1 0 0 0 0
34 36 1 0 0 0 0
34 57 1 0 0 0 0
36 58 1 0 0 0 0
37 59 1 0 0 0 0
37 60 1 0 0 0 0
37 61 1 0 0 0 0
38 62 1 0 0 0 0
38 63 1 0 0 0 0
38 64 1 0 0 0 0
39 65 1 0 0 0 0
39 66 1 0 0 0 0
39 67 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
(2S,10R,11R)-2-hydroxy-4,6-dimethoxy-10-(4-methoxyphenyl)-11-phenyl-9-oxa-14,19-diazapentacyclo[10.7.0.02,10.03,8.014,18]nonadeca-1(12),3(8),4,6,18-pentaen-13-one
4.2 InChl
InChI=1S/C31H28N2O6/c1-36-20-13-11-19(12-14-20)31-26(18-8-5-4-6-9-18)25-28(32-24-10-7-15-33(24)29(25)34)30(31,35)27-22(38-3)16-21(37-2)17-23(27)39-31/h4-6,8-9,11-14,16-17,26,35H,7,10,15H2,1-3H3/t26-,30+,31+/m1/s1
4.3 InChlKey
YCIPQJTZJGUXND-JZRGNDHQSA-N
4.4 Canonical SMILES
COC1=CC=C(C=C1)C23C(C4=C(C2(C5=C(O3)C=C(C=C5OC)OC)O)N=C6CCCN6C4=O)C7=CC=CC=C7
4.5 lsomeric SMILES
COC1=CC=C(C=C1)[C@]23[C@@H](C4=C([C@]2(C5=C(O3)C=C(C=C5OC)OC)O)N=C6CCCN6C4=O)C7=CC=CC=C7
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病