3. 结构
3.1 二维结构
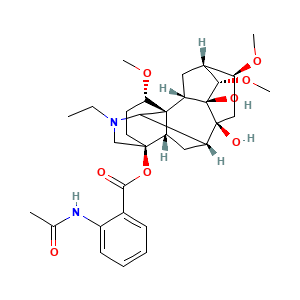
3.2 三维结构
-1
-2
-3
86 92 0 1 0 0 0 0 0999 V2000
-3.1209 -1.9047 -2.0621 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.6705 -3.0167 0.3163 O 0 0 0 0 0 0 0 0 0 0 0 0
2.2780 0.1489 -1.5907 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.1495 3.2725 -0.6064 O 0 0 0 0 0 0 0 0 0 0 0 0
-5.5833 -2.1108 -0.2872 O 0 0 0 0 0 0 0 0 0 0 0 0
-5.7094 -0.4430 2.3146 O 0 0 0 0 0 0 0 0 0 0 0 0
3.7456 1.6512 -0.6484 O 0 0 0 0 0 0 0 0 0 0 0 0
7.2939 -0.4903 2.3085 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.0228 0.9688 1.1920 N 0 0 2 0 0 0 0 0 0 0 0 0
5.4997 0.2720 0.9835 N 0 0 0 0 0 0 0 0 0 0 0 0
-1.4341 0.9144 -0.9452 C 0 0 1 0 0 0 0 0 0 0 0 0
-2.9817 0.4077 -1.3167 C 0 0 1 0 0 0 0 0 0 0 0 0
-1.2634 0.5320 0.5266 C 0 0 1 0 0 0 0 0 0 0 0 0
-0.1496 0.0589 -1.4528 C 0 0 2 0 0 0 0 0 0 0 0 0
-1.2924 -1.0485 0.3181 C 0 0 1 0 0 0 0 0 0 0 0 0
-3.4760 -1.0678 -0.9668 C 0 0 2 0 0 0 0 0 0 0 0 0
-2.7636 -1.5865 0.3670 C 0 0 1 0 0 0 0 0 0 0 0 0
-0.0532 -1.2708 -0.5578 C 0 0 0 0 0 0 0 0 0 0 0 0
1.1234 0.8598 -1.1279 C 0 0 1 0 0 0 0 0 0 0 0 0
-1.3299 2.4157 -1.3960 C 0 0 2 0 0 0 0 0 0 0 0 0
-4.1065 1.2414 -0.6183 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.0589 0.2556 0.0884 C 0 0 1 0 0 0 0 0 0 0 0 0
-4.9885 -0.9487 -0.8275 C 0 0 2 0 0 0 0 0 0 0 0 0
-3.5447 -1.2141 1.6443 C 0 0 0 0 0 0 0 0 0 0 0 0
1.2196 1.0963 0.4090 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.5752 -0.0708 1.5200 C 0 0 2 0 0 0 0 0 0 0 0 0
1.1329 2.1663 -1.9132 C 0 0 0 0 0 0 0 0 0 0 0 0
0.0380 3.0875 -1.4441 C 0 0 0 0 0 0 0 0 0 0 0 0
0.1851 0.3630 2.4994 C 0 0 0 0 0 0 0 0 0 0 0 0
1.1471 1.1860 3.3392 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.6265 4.3754 -1.3632 C 0 0 0 0 0 0 0 0 0 0 0 0
-7.0009 -2.0939 -0.4248 C 0 0 0 0 0 0 0 0 0 0 0 0
3.4605 0.5327 -1.0471 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.4773 -0.1812 3.6873 C 0 0 0 0 0 0 0 0 0 0 0 0
4.4015 -0.6147 -0.9955 C 0 0 0 0 0 0 0 0 0 0 0 0
5.3759 -0.7115 -0.0020 C 0 0 0 0 0 0 0 0 0 0 0 0
4.2957 -1.6091 -1.9680 C 0 0 0 0 0 0 0 0 0 0 0 0
6.2444 -1.8030 0.0188 C 0 0 0 0 0 0 0 0 0 0 0 0
5.1643 -2.7004 -1.9470 C 0 0 0 0 0 0 0 0 0 0 0 0
6.1386 -2.7973 -0.9537 C 0 0 0 0 0 0 0 0 0 0 0 0
6.4149 0.3227 2.0413 C 0 0 0 0 0 0 0 0 0 0 0 0
6.2198 1.5552 2.8881 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.0750 0.5471 -2.4032 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.0867 0.8956 1.1385 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.2241 -0.1995 -2.5146 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.9373 -1.5077 1.2525 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.2019 -2.1493 -1.1941 H 0 0 0 0 0 0 0 0 0 0 0 0
0.8805 -1.4101 -0.0078 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.6979 2.4151 -2.4332 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.7604 1.9708 0.1107 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.6589 1.7921 -1.3904 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.0712 0.6728 0.1331 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.4312 -0.7365 -1.8111 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.0679 -2.1147 1.9990 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.8508 -0.9871 2.4636 H 0 0 0 0 0 0 0 0 0 0 0 0
1.6558 2.0781 0.6296 H 0 0 0 0 0 0 0 0 0 0 0 0
1.9328 0.3685 0.8172 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.1547 0.8474 1.9490 H 0 0 0 0 0 0 0 0 0 0 0 0
1.0037 1.9499 -2.9828 H 0 0 0 0 0 0 0 0 0 0 0 0
2.0826 2.7057 -1.8550 H 0 0 0 0 0 0 0 0 0 0 0 0
0.2846 3.5101 -0.4619 H 0 0 0 0 0 0 0 0 0 0 0 0
0.0094 3.9537 -2.1166 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.5872 -1.5868 -2.8537 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.2203 -3.3179 1.1240 H 0 0 0 0 0 0 0 0 0 0 0 0
-0.7640 0.3140 3.0468 H 0 0 0 0 0 0 0 0 0 0 0 0
0.5766 -0.6580 2.4287 H 0 0 0 0 0 0 0 0 0 0 0 0
1.1918 0.7797 4.3556 H 0 0 0 0 0 0 0 0 0 0 0 0
0.8166 2.2277 3.4155 H 0 0 0 0 0 0 0 0 0 0 0 0
2.1676 1.1760 2.9439 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.3172 4.9401 -0.7300 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.1800 4.0401 -2.2460 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.8170 5.0502 -1.6522 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.3941 -3.0182 0.0071 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.2843 -2.0589 -1.4813 H 0 0 0 0 0 0 0 0 0 0 0 0
-7.4400 -1.2494 0.1131 H 0 0 0 0 0 0 0 0 0 0 0 0
-6.3681 -0.4840 4.2451 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.3194 0.8884 3.8588 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.6286 -0.7546 4.0708 H 0 0 0 0 0 0 0 0 0 0 0 0
3.5573 -1.5573 -2.7637 H 0 0 0 0 0 0 0 0 0 0 0 0
7.0245 -1.9550 0.7504 H 0 0 0 0 0 0 0 0 0 0 0 0
5.0843 -3.4732 -2.7059 H 0 0 0 0 0 0 0 0 0 0 0 0
4.8401 1.0436 0.9269 H 0 0 0 0 0 0 0 0 0 0 0 0
6.8147 -3.6473 -0.9392 H 0 0 0 0 0 0 0 0 0 0 0 0
6.9223 1.5445 3.7260 H 0 0 0 0 0 0 0 0 0 0 0 0
5.1996 1.5780 3.2818 H 0 0 0 0 0 0 0 0 0 0 0 0
6.3987 2.4496 2.2846 H 0 0 0 0 0 0 0 0 0 0 0 0
1 16 1 0 0 0 0
1 63 1 0 0 0 0
2 17 1 0 0 0 0
2 64 1 0 0 0 0
3 19 1 0 0 0 0
3 33 1 0 0 0 0
4 20 1 0 0 0 0
4 31 1 0 0 0 0
5 23 1 0 0 0 0
5 32 1 0 0 0 0
6 26 1 0 0 0 0
6 34 1 0 0 0 0
7 33 2 0 0 0 0
8 41 2 0 0 0 0
9 13 1 0 0 0 0
9 25 1 0 0 0 0
9 29 1 0 0 0 0
10 36 1 0 0 0 0
10 41 1 0 0 0 0
10 82 1 0 0 0 0
11 12 1 0 0 0 0
11 13 1 0 0 0 0
11 14 1 0 0 0 0
11 20 1 0 0 0 0
12 16 1 0 0 0 0
12 21 1 0 0 0 0
12 43 1 0 0 0 0
13 15 1 0 0 0 0
13 44 1 0 0 0 0
14 18 1 0 0 0 0
14 19 1 0 0 0 0
14 45 1 0 0 0 0
15 17 1 0 0 0 0
15 18 1 0 0 0 0
15 46 1 0 0 0 0
16 17 1 0 0 0 0
16 23 1 0 0 0 0
17 24 1 0 0 0 0
18 47 1 0 0 0 0
18 48 1 0 0 0 0
19 25 1 0 0 0 0
19 27 1 0 0 0 0
20 28 1 0 0 0 0
20 49 1 0 0 0 0
21 22 1 0 0 0 0
21 50 1 0 0 0 0
21 51 1 0 0 0 0
22 23 1 0 0 0 0
22 26 1 0 0 0 0
22 52 1 0 0 0 0
23 53 1 0 0 0 0
24 26 1 0 0 0 0
24 54 1 0 0 0 0
24 55 1 0 0 0 0
25 56 1 0 0 0 0
25 57 1 0 0 0 0
26 58 1 0 0 0 0
27 28 1 0 0 0 0
27 59 1 0 0 0 0
27 60 1 0 0 0 0
28 61 1 0 0 0 0
28 62 1 0 0 0 0
29 30 1 0 0 0 0
29 65 1 0 0 0 0
29 66 1 0 0 0 0
30 67 1 0 0 0 0
30 68 1 0 0 0 0
30 69 1 0 0 0 0
31 70 1 0 0 0 0
31 71 1 0 0 0 0
31 72 1 0 0 0 0
32 73 1 0 0 0 0
32 74 1 0 0 0 0
32 75 1 0 0 0 0
33 35 1 0 0 0 0
34 76 1 0 0 0 0
34 77 1 0 0 0 0
34 78 1 0 0 0 0
35 36 1 0 0 0 0
35 37 2 0 0 0 0
36 38 2 0 0 0 0
37 39 1 0 0 0 0
37 79 1 0 0 0 0
38 40 1 0 0 0 0
38 80 1 0 0 0 0
39 40 2 0 0 0 0
39 81 1 0 0 0 0
40 83 1 0 0 0 0
41 42 1 0 0 0 0
42 84 1 0 0 0 0
42 85 1 0 0 0 0
42 86 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
[(1S,2S,3S,4S,5R,6S,8S,9R,13S,16S,17S)-11-ethyl-3,8-dihydroxy-4,6,16-trimethoxy-11-azahexacyclo[7.7.2.12,5.01,10.03,8.013,17]nonadecan-13-yl] 2-acetamidobenzoate
4.2 InChl
InChI=1S/C32H44N2O8/c1-6-34-16-29(42-28(36)18-9-7-8-10-21(18)33-17(2)35)12-11-25(40-4)31-23(29)14-20(26(31)34)30(37)15-22(39-3)19-13-24(31)32(30,38)27(19)41-5/h7-10,19-20,22-27,37-38H,6,11-16H2,1-5H3,(H,33,35)/t19-,20-,22+,23-,24+,25+,26?,27+,29-,30+,31+,32+/m1/s1
4.3 InChlKey
NWBWCXBPKTTZNQ-MUVXNFNYSA-N
4.4 Canonical SMILES
CCN1CC2(CCC(C34C2CC(C31)C5(CC(C6CC4C5(C6OC)O)OC)O)OC)OC(=O)C7=CC=CC=C7NC(=O)C
4.5 lsomeric SMILES
CCN1C[C@@]2(CC[C@@H]([C@@]34[C@@H]2C[C@H](C31)[C@]5(C[C@@H]([C@H]6C[C@@H]4[C@@]5([C@H]6OC)O)OC)O)OC)OC(=O)C7=CC=CC=C7NC(=O)C
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病